Substrate Level Phosphorylation Occurs In
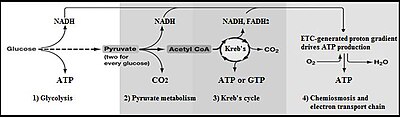
Summary of aerobic respiration
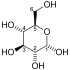
Glycolysis is the metabolic pathway that converts glucose (C6H12Osix ) into pyruvate (CH3COCO2H). The complimentary energy released in this process is used to course the high-free energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH).[1] Glycolysis is a sequence of ten reactions catalyzed by enzymes. Capture of bond energy of carbohydrates. Storage of ATP

Summary of the ten reactions of the glycolysis pathway
Glycolysis is a metabolic pathway that does not require oxygen (In anaerobic conditions pyruvate is converted to lactic acid). The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway.[2] Indeed, the reactions that make up glycolysis and its parallel pathway, the pentose phosphate pathway, occur in the oxygen-gratuitous conditions of the Archean oceans, also in the absence of enzymes, catalyzed by metal.[3]
In most organisms, glycolysis occurs in the liquid part of cells, the cytosol. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP) pathway, which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis as well refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.[4]
The glycolysis pathway can exist separated into 2 phases:[5]
- Investment phase – wherein ATP is consumed
- Yield phase – wherein more ATP is produced than originally consumed
Overview [edit]
The overall reaction of glycolysis is:

+ 2 [NAD]+
+ 2 [ADP]
+ 2 [P]i
2 × 
+ ii [NADH]
+ two H+
+ 2 [ATP]
+ 2 H2O

Glycolysis pathway overview.
The utilise of symbols in this equation makes it appear unbalanced with respect to oxygen atoms, hydrogen atoms, and charges. Atom balance is maintained by the 2 phosphate (Pi) groups:[6]
- Each exists in the form of a hydrogen phosphate anion ([HPO4]two− ), dissociating to contribute 2H+ overall
- Each liberates an oxygen atom when it binds to an adenosine diphosphate (ADP) molecule, contributing 2O overall
Charges are balanced by the departure between ADP and ATP. In the cellular surround, all three hydroxyl groups of ADP dissociate into −O− and H+, giving ADP3−, and this ion tends to exist in an ionic bail with Mgii+, giving ADPMg−. ATP behaves identically except that it has four hydroxyl groups, giving ATPMg2−. When these differences forth with the true charges on the 2 phosphate groups are considered together, the net charges of −4 on each side are balanced.
For simple fermentations, the metabolism of one molecule of glucose to two molecules of pyruvate has a cyberspace yield of two molecules of ATP. Nearly cells will then carry out further reactions to "repay" the used NAD+ and produce a final product of ethanol or lactic acid. Many bacteria use inorganic compounds every bit hydrogen acceptors to regenerate the NAD+.
Cells performing aerobic respiration synthesize much more than ATP, but not as function of glycolysis. These further aerobic reactions utilize pyruvate, and NADH + H+ from glycolysis. Eukaryotic aerobic respiration produces approximately 34 additional molecules of ATP for each glucose molecule, still most of these are produced by a mechanism vastly dissimilar from the substrate-level phosphorylation in glycolysis.
The lower-energy product, per glucose, of anaerobic respiration relative to aerobic respiration, results in greater flux through the pathway nether hypoxic (low-oxygen) conditions, unless alternative sources of anaerobically oxidizable substrates, such as fat acids, are found.
| Metabolism of common monosaccharides, including glycolysis, gluconeogenesis, glycogenesis and glycogenolysis |
|---|
| |
History [edit]
The pathway of glycolysis as it is known today took well-nigh 100 years to fully elucidate.[seven] The combined results of many smaller experiments were required in order to understand the pathway as a whole.
The starting time steps in understanding glycolysis began in the nineteenth century with the wine industry. For economic reasons, the French wine industry sought to investigate why wine sometimes turned distasteful, instead of fermenting into alcohol. French scientist Louis Pasteur researched this upshot during the 1850s, and the results of his experiments began the long road to elucidating the pathway of glycolysis.[viii] His experiments showed that fermentation occurs by the activity of living microorganisms, yeasts, and that yeast's glucose consumption decreased nether aerobic conditions of fermentation, in comparison to anaerobic conditions (the Pasteur effect).[9]

Eduard Buchner. Discovered jail cell-free fermentation.
Insight into the component steps of glycolysis were provided past the non-cellular fermentation experiments of Eduard Buchner during the 1890s.[10] [11] Buchner demonstrated that the conversion of glucose to ethanol was possible using a not-living extract of yeast, due to the activity of enzymes in the extract.[12] : 135–148 This experiment not only revolutionized biochemistry, just as well allowed later on scientists to clarify this pathway in a more than controlled laboratory setting. In a series of experiments (1905-1911), scientists Arthur Harden and William Young discovered more pieces of glycolysis.[13] They discovered the regulatory furnishings of ATP on glucose consumption during booze fermentation. They besides shed lite on the role of 1 compound equally a glycolysis intermediate: fructose 1,half dozen-bisphosphate.[12] : 151–158
The elucidation of fructose 1,half dozen-bisphosphate was achieved past measuring COtwo levels when yeast juice was incubated with glucose. CO2 product increased rapidly and then slowed down. Harden and Young noted that this process would restart if an inorganic phosphate (Pi) was added to the mixture. Harden and Young deduced that this process produced organic phosphate esters, and further experiments allowed them to excerpt fructose diphosphate (F-1,6-DP).
Arthur Harden and William Young along with Nick Sheppard determined, in a second experiment, that a heat-sensitive high-molecular-weight subcellular fraction (the enzymes) and a heat-insensitive low-molecular-weight cytoplasm fraction (ADP, ATP and NAD+ and other cofactors) are required together for fermentation to proceed. This experiment begun past observing that dialyzed (purified) yeast juice could not ferment or even create a sugar phosphate. This mixture was rescued with the addition of undialyzed yeast extract that had been boiled. Humid the yeast excerpt renders all proteins inactive (as it denatures them). The ability of boiled extract plus dialyzed juice to complete fermentation suggests that the cofactors were non-protein in character.[xiii]

Otto Meyerhof. Ane of the main scientists involved in completing the puzzle of glycolysis
In the 1920s Otto Meyerhof was able to link together some of the many individual pieces of glycolysis discovered by Buchner, Harden, and Immature. Meyerhof and his team were able to excerpt different glycolytic enzymes from muscle tissue, and combine them to artificially create the pathway from glycogen to lactic acid.[14] [15]
In ane newspaper, Meyerhof and scientist Renate Junowicz-Kockolaty investigated the reaction that splits fructose 1,6-diphosphate into the two triose phosphates. Previous work proposed that the dissever occurred via 1,three-diphosphoglyceraldehyde plus an oxidizing enzyme and cozymase. Meyerhoff and Junowicz establish that the equilibrium constant for the isomerase and aldoses reaction were not affected by inorganic phosphates or any other cozymase or oxidizing enzymes. They farther removed diphosphoglyceraldehyde equally a possible intermediate in glycolysis.[15]
With all of these pieces available by the 1930s, Gustav Embden proposed a detailed, step-by-stride outline of that pathway we now know as glycolysis.[16] The biggest difficulties in determining the intricacies of the pathway were due to the very short lifetime and low steady-state concentrations of the intermediates of the fast glycolytic reactions. By the 1940s, Meyerhof, Embden and many other biochemists had finally completed the puzzle of glycolysis.[15] The agreement of the isolated pathway has been expanded in the subsequent decades, to include further details of its regulation and integration with other metabolic pathways.
Sequence of reactions [edit]
Summary of reactions [edit]
![]()

![]()
![]()

![]()

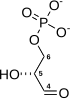
![]()
2 × 
![]()
2 × 
![]()
2 × 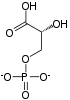
![]()
2 × 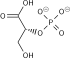
![]()
2 × 
![]()
2 × ![]()
Preparatory phase [edit]
The offset v steps of Glycolysis are regarded equally the preparatory (or investment) phase, since they consume energy to convert the glucose into two iii-carbon saccharide phosphates[5] (G3P).
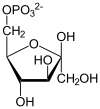
The first pace is phosphorylation of glucose by a family of enzymes chosen hexokinases to form glucose 6-phosphate (G6P). This reaction consumes ATP, only it acts to keep the glucose concentration depression, promoting continuous transport of glucose into the cell through the plasma membrane transporters. In addition, information technology blocks the glucose from leaking out – the prison cell lacks transporters for G6P, and free diffusion out of the cell is prevented due to the charged nature of G6P. Glucose may alternatively exist formed from the phosphorolysis or hydrolysis of intracellular starch or glycogen.
In animals, an isozyme of hexokinase called glucokinase is also used in the liver, which has a much lower affinity for glucose (Kthou in the vicinity of normal glycemia), and differs in regulatory backdrop. The different substrate affinity and alternating regulation of this enzyme are a reflection of the role of the liver in maintaining claret carbohydrate levels.
Cofactors: Mgii+
G6P is then rearranged into fructose six-phosphate (F6P) by glucose phosphate isomerase. Fructose can too enter the glycolytic pathway by phosphorylation at this point.
The modify in construction is an isomerization, in which the G6P has been converted to F6P. The reaction requires an enzyme, phosphoglucose isomerase, to go on. This reaction is freely reversible nether normal cell conditions. However, it is often driven frontward considering of a depression concentration of F6P, which is constantly consumed during the next step of glycolysis. Under weather of high F6P concentration, this reaction readily runs in reverse. This phenomenon can exist explained through Le Chatelier's Principle. Isomerization to a keto sugar is necessary for carbanion stabilization in the fourth reaction step (beneath).
The energy expenditure of another ATP in this step is justified in two ways: The glycolytic process (up to this step) becomes irreversible, and the energy supplied destabilizes the molecule. Because the reaction catalyzed by phosphofructokinase 1 (PFK-one) is coupled to the hydrolysis of ATP (an energetically favorable step) it is, in essence, irreversible, and a unlike pathway must be used to do the reverse conversion during gluconeogenesis. This makes the reaction a key regulatory point (meet below). This is too the rate-limiting step.
Furthermore, the second phosphorylation event is necessary to allow the germination of 2 charged groups (rather than only one) in the subsequent step of glycolysis, ensuring the prevention of free diffusion of substrates out of the jail cell.
The aforementioned reaction tin also exist catalyzed by pyrophosphate-dependent phosphofructokinase (PFP or PPi-PFK), which is found in most plants, some leaner, archea, and protists, but not in animals. This enzyme uses pyrophosphate (PPi) as a phosphate donor instead of ATP. Information technology is a reversible reaction, increasing the flexibility of glycolytic metabolism.[17] A rarer ADP-dependent PFK enzyme variant has been identified in archaean species.[18]
Cofactors: Mgtwo+
Destabilizing the molecule in the previous reaction allows the hexose ring to be split past aldolase into two triose sugars: dihydroxyacetone phosphate (a ketose), and glyceraldehyde 3-phosphate (an aldose). There are two classes of aldolases: grade I aldolases, present in animals and plants, and class II aldolases, present in fungi and leaner; the two classes employ different mechanisms in cleaving the ketose ring.
Electrons delocalized in the carbon-carbon bail cleavage associate with the alcohol grouping. The resulting carbanion is stabilized by the construction of the carbanion itself via resonance charge distribution and by the presence of a charged ion prosthetic grouping.
Triosephosphate isomerase apace interconverts dihydroxyacetone phosphate with glyceraldehyde 3-phosphate (GADP) that gain further into glycolysis. This is advantageous, every bit information technology directs dihydroxyacetone phosphate downwardly the same pathway as glyceraldehyde 3-phosphate, simplifying regulation.
Pay-off phase [edit]
The second half of glycolysis is known as the pay-off phase, characterised past a net gain of the energy-rich molecules ATP and NADH.[5] Since glucose leads to ii triose sugars in the preparatory phase, each reaction in the pay-off stage occurs twice per glucose molecule. This yields 2 NADH molecules and 4 ATP molecules, leading to a net proceeds of 2 NADH molecules and 2 ATP molecules from the glycolytic pathway per glucose.
The aldehyde groups of the triose sugars are oxidised, and inorganic phosphate is added to them, forming 1,3-bisphosphoglycerate.
The hydrogen is used to reduce two molecules of NAD+, a hydrogen carrier, to requite NADH + H+ for each triose.
Hydrogen cantlet balance and charge balance are both maintained because the phosphate (Pi) group really exists in the form of a hydrogen phosphate anion (HPO 2− four ),[6] which dissociates to contribute the extra H+ ion and gives a cyberspace charge of -iii on both sides.
Hither, arsenate ([AsOiv]3− ), an anion akin to inorganic phosphate may replace phosphate equally a substrate to form 1-arseno-3-phosphoglycerate. This, still, is unstable and readily hydrolyzes to grade 3-phosphoglycerate, the intermediate in the next pace of the pathway. Every bit a consequence of bypassing this step, the molecule of ATP generated from 1-3 bisphosphoglycerate in the next reaction will not be fabricated, even though the reaction proceeds. As a result, arsenate is an uncoupler of glycolysis.[19]
This step is the enzymatic transfer of a phosphate group from 1,3-bisphosphoglycerate to ADP past phosphoglycerate kinase, forming ATP and 3-phosphoglycerate. At this step, glycolysis has reached the interruption-even indicate: two molecules of ATP were consumed, and 2 new molecules take now been synthesized. This step, i of the ii substrate-level phosphorylation steps, requires ADP; thus, when the prison cell has plenty of ATP (and petty ADP), this reaction does not occur. Because ATP decays relatively quickly when it is non metabolized, this is an important regulatory point in the glycolytic pathway.
ADP actually exists as ADPMg−, and ATP every bit ATPMgii−, balancing the charges at −5 both sides.
Cofactors: Mg2+
Phosphoglycerate mutase isomerises 3-phosphoglycerate into ii-phosphoglycerate.
Enolase adjacent converts 2-phosphoglycerate to phosphoenolpyruvate. This reaction is an emptying reaction involving an E1cB mechanism.
Cofactors: two Mg2+, one "conformational" ion to coordinate with the carboxylate grouping of the substrate, and ane "catalytic" ion that participates in the dehydration.
A final substrate-level phosphorylation at present forms a molecule of pyruvate and a molecule of ATP past means of the enzyme pyruvate kinase. This serves every bit an additional regulatory step, similar to the phosphoglycerate kinase step.
Cofactors: Mgtwo+
Biochemical logic [edit]
The existence of more than than i point of regulation indicates that intermediates between those points enter and leave the glycolysis pathway by other processes. For example, in the kickoff regulated step, hexokinase converts glucose into glucose-6-phosphate. Instead of continuing through the glycolysis pathway, this intermediate can exist converted into glucose storage molecules, such as glycogen or starch. The reverse reaction, breaking down, e.g., glycogen, produces mainly glucose-half-dozen-phosphate; very little free glucose is formed in the reaction. The glucose-half-dozen-phosphate so produced tin can enter glycolysis after the first control point.
In the second regulated step (the third step of glycolysis), phosphofructokinase converts fructose-half dozen-phosphate into fructose-1,six-bisphosphate, which then is converted into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. The dihydroxyacetone phosphate tin can exist removed from glycolysis past conversion into glycerol-3-phosphate, which tin be used to form triglycerides.[20] Conversely, triglycerides tin can be broken down into fatty acids and glycerol; the latter, in turn, can be converted into dihydroxyacetone phosphate, which tin enter glycolysis after the second control indicate.
Free free energy changes [edit]
| Compound | Concentration / mM |
|---|---|
| Glucose | 5.0 |
| Glucose-6-phosphate | 0.083 |
| Fructose-6-phosphate | 0.014 |
| Fructose-1,half-dozen-bisphosphate | 0.031 |
| Dihydroxyacetone phosphate | 0.14 |
| Glyceraldehyde-3-phosphate | 0.019 |
| 1,three-Bisphosphoglycerate | 0.001 |
| 2,3-Bisphosphoglycerate | 4.0 |
| three-Phosphoglycerate | 0.12 |
| ii-Phosphoglycerate | 0.03 |
| Phosphoenolpyruvate | 0.023 |
| Pyruvate | 0.051 |
| ATP | one.85 |
| ADP | 0.14 |
| Pi | 1.0 |
The alter in complimentary energy, ΔG, for each step in the glycolysis pathway can exist calculated using ΔK = Δ1000°' + RTln Q, where Q is the reaction quotient. This requires knowing the concentrations of the metabolites. All of these values are available for erythrocytes, with the exception of the concentrations of NAD+ and NADH. The ratio of NAD+ to NADH in the cytoplasm is approximately 1000, which makes the oxidation of glyceraldehyde-3-phosphate (step 6) more favourable.
Using the measured concentrations of each step, and the standard gratuitous energy changes, the actual free energy alter can be calculated. (Neglecting this is very common - the delta G of ATP hydrolysis in cells is not the standard gratuitous energy change of ATP hydrolysis quoted in textbooks).
| Step | Reaction | ΔG°' / (kJ/mol) | ΔG / (kJ/mol) |
|---|---|---|---|
| ane | Glucose + ATP4− → Glucose-vi-phosphateii− + ADP3− + H+ | −xvi.vii | −34 |
| 2 | Glucose-half-dozen-phosphate2− → Fructose-six-phosphate2− | 1.67 | −2.nine |
| 3 | Fructose-6-phosphate2− + ATPfour− → Fructose-1,6-bisphosphate4− + ADPiii− + H+ | −14.2 | −19 |
| 4 | Fructose-1,6-bisphosphateiv− → Dihydroxyacetone phosphate2− + Glyceraldehyde-3-phosphate2− | 23.ix | −0.23 |
| 5 | Dihydroxyacetone phosphate2− → Glyceraldehyde-3-phosphate2− | 7.56 | ii.4 |
| 6 | Glyceraldehyde-3-phosphate2− + Pi ii− + NAD+ → 1,3-Bisphosphoglycerate4− + NADH + H+ | 6.xxx | −i.29 |
| 7 | one,3-Bisphosphoglycerate4− + ADP3− → three-Phosphoglyceratethree− + ATP4− | −xviii.9 | 0.09 |
| eight | 3-Phosphoglycerate3− → 2-Phosphoglyceratethree− | four.four | 0.83 |
| 9 | 2-Phosphoglycerate3− → Phosphoenolpyruvate3− + HiiO | 1.8 | one.1 |
| 10 | Phosphoenolpyruvatethree− + ADPiii− + H+ → Pyruvate− + ATPiv− | −31.vii | −23.0 |
From measuring the physiological concentrations of metabolites in an erythrocyte information technology seems that most 7 of the steps in glycolysis are in equilibrium for that cell blazon. 3 of the steps — the ones with large negative gratuitous energy changes — are not in equilibrium and are referred to as irreversible; such steps are oftentimes field of study to regulation.
Step 5 in the figure is shown backside the other steps, because that step is a side-reaction that tin decrease or increase the concentration of the intermediate glyceraldehyde-three-phosphate. That compound is converted to dihydroxyacetone phosphate past the enzyme triose phosphate isomerase, which is a catalytically perfect enzyme; its rate is then fast that the reaction can be assumed to be in equilibrium. The fact that ΔG is non zero indicates that the actual concentrations in the erythrocyte are non accurately known.
Regulation [edit]
The enzymes that catalyse glycolysis are regulated via a range of biological mechanisms in order to control overall flux though the pathway. This is vital for both homeostatsis in a static environment, and metabolic adaptation to a changing environment or need.[22] The details of regulation for some enzymes are highly conserved between species, whereas others vary widely.[23] [24]
- Cistron Expression: Firstly, the cellular concentrations of glycolytic enzymes are modulated via regulation of factor expression via transcription factors,[25] with several glycolysis enzymes themselves acting equally regulatory poly peptide kinases in the nucleus.[26]
- Allosteric inhibition and activation by metabolites: In particular end-product inhibition of rate limiting enzymes by metabolites such equally ATP serves as negative feedback regulation of the pathway.[23] [27]
- Allosteric inhibition and activation by Protein-poly peptide interactions (PPI).[28] Indeed, some proteins interact with and regulate multiple glycolytic enzymes.[29]
- Mail-translational modification (PTM).[30] In particular, phosphorylation and dephosphorylation is a key machinery of regulation of pyruvate kinase in the liver.
- Localization[27]
Regulation past insulin in animals [edit]
In animals, regulation of claret glucose levels by the pancreas in conjunction with the liver is a vital part of homeostasis. The beta cells in the pancreatic islets are sensitive to the blood glucose concentration.[31] A rise in the claret glucose concentration causes them to release insulin into the blood, which has an issue particularly on the liver, but as well on fatty and muscle cells, causing these tissues to remove glucose from the claret. When the blood sugar falls the pancreatic beta cells finish insulin production, simply, instead, stimulate the neighboring pancreatic blastoff cells to release glucagon into the blood.[31] This, in plough, causes the liver to release glucose into the blood by breaking down stored glycogen, and past means of gluconeogenesis. If the autumn in the claret glucose level is particularly rapid or severe, other glucose sensors cause the release of epinephrine from the adrenal glands into the claret. This has the aforementioned action as glucagon on glucose metabolism, but its effect is more pronounced.[31] In the liver glucagon and epinephrine crusade the phosphorylation of the key, rate limiting enzymes of glycolysis, fat acid synthesis, cholesterol synthesis, gluconeogenesis, and glycogenolysis. Insulin has the opposite consequence on these enzymes.[32] The phosphorylation and dephosphorylation of these enzymes (ultimately in response to the glucose level in the blood) is the dominant fashion by which these pathways are controlled in the liver, fat, and musculus cells. Thus the phosphorylation of phosphofructokinase inhibits glycolysis, whereas its dephosphorylation through the action of insulin stimulates glycolysis.[32]
Regulation of the rate limiting enzymes [edit]
The three regulatory enzymes are hexokinase (or glucokinase in the liver), phosphofructokinase, and pyruvate kinase. The flux through the glycolytic pathway is adapted in response to weather both within and exterior the cell. The internal factors that regulate glycolysis practise so primarily to provide ATP in acceptable quantities for the cell's needs. The external factors human action primarily on the liver, fat tissue, and muscles, which tin can remove large quantities of glucose from the blood after meals (thus preventing hyperglycemia by storing the excess glucose as fat or glycogen, depending on the tissue blazon). The liver is likewise capable of releasing glucose into the claret between meals, during fasting, and exercise thus preventing hypoglycemia past means of glycogenolysis and gluconeogenesis. These latter reactions coincide with the halting of glycolysis in the liver.
In add-on hexokinase and glucokinase act independently of the hormonal effects as controls at the entry points of glucose into the cells of dissimilar tissues. Hexokinase responds to the glucose-6-phosphate (G6P) level in the prison cell, or, in the case of glucokinase, to the blood sugar level in the blood to impart entirely intracellular controls of the glycolytic pathway in different tissues (meet below).[32]
When glucose has been converted into G6P by hexokinase or glucokinase, information technology can either exist converted to glucose-ane-phosphate (G1P) for conversion to glycogen, or it is alternatively converted by glycolysis to pyruvate, which enters the mitochondrion where information technology is converted into acetyl-CoA and then into citrate. Excess citrate is exported from the mitochondrion back into the cytosol, where ATP citrate lyase regenerates acetyl-CoA and oxaloacetate (OAA). The acetyl-CoA is then used for fatty acid synthesis and cholesterol synthesis, two important ways of utilizing backlog glucose when its concentration is high in blood. The charge per unit limiting enzymes catalyzing these reactions perform these functions when they take been dephosphorylated through the action of insulin on the liver cells. Between meals, during fasting, practise or hypoglycemia, glucagon and epinephrine are released into the blood. This causes liver glycogen to be converted back to G6P, and so converted to glucose by the liver-specific enzyme glucose 6-phosphatase and released into the claret. Glucagon and epinephrine also stimulate gluconeogenesis, which coverts not-saccharide substrates into G6P, which joins the G6P derived from glycogen, or substitutes for information technology when the liver glycogen store have been depleted. This is disquisitional for brain function, since the encephalon utilizes glucose equally an free energy source under virtually conditions.[33] The simultaneously phosphorylation of, particularly, phosphofructokinase, only likewise, to a certain extent pyruvate kinase, prevents glycolysis occurring at the same time as gluconeogenesis and glycogenolysis.
Hexokinase and glucokinase [edit]

All cells contain the enzyme hexokinase, which catalyzes the conversion of glucose that has entered the cell into glucose-six-phosphate (G6P). Since the cell membrane is impervious to G6P, hexokinase substantially acts to ship glucose into the cells from which it can and so no longer escape. Hexokinase is inhibited by loftier levels of G6P in the prison cell. Thus the rate of entry of glucose into cells partially depends on how fast G6P tin be disposed of by glycolysis, and past glycogen synthesis (in the cells which shop glycogen, namely liver and muscles).[32] [34]
Glucokinase, unlike hexokinase, is non inhibited by G6P. It occurs in liver cells, and volition only phosphorylate the glucose entering the cell to form glucose-half-dozen-phosphate (G6P), when the glucose in the blood is abundant. This being the commencement step in the glycolytic pathway in the liver, it therefore imparts an additional layer of control of the glycolytic pathway in this organ.[32]
Phosphofructokinase [edit]
Phosphofructokinase is an important control point in the glycolytic pathway, since it is ane of the irreversible steps and has key allosteric effectors, AMP and fructose ii,six-bisphosphate (F2,6BP).
Fructose 2,6-bisphosphate (F2,6BP) is a very stiff activator of phosphofructokinase (PFK-1) that is synthesized when F6P is phosphorylated by a second phosphofructokinase (PFK2). In the liver, when blood sugar is low and glucagon elevates cAMP, PFK2 is phosphorylated by protein kinase A. The phosphorylation inactivates PFK2, and some other domain on this protein becomes active as fructose bisphosphatase-2, which converts F2,6BP back to F6P. Both glucagon and epinephrine cause loftier levels of cAMP in the liver. The result of lower levels of liver fructose-ii,6-bisphosphate is a decrease in activity of phosphofructokinase and an increase in action of fructose 1,6-bisphosphatase, then that gluconeogenesis (in essence, "glycolysis in reverse") is favored. This is consequent with the role of the liver in such situations, since the response of the liver to these hormones is to release glucose to the blood.
ATP competes with AMP for the allosteric effector site on the PFK enzyme. ATP concentrations in cells are much higher than those of AMP, typically 100-fold higher,[35] merely the concentration of ATP does not change more than virtually x% nether physiological atmospheric condition, whereas a x% drop in ATP results in a 6-fold increase in AMP.[36] Thus, the relevance of ATP as an allosteric effector is questionable. An increase in AMP is a upshot of a subtract in energy charge in the cell.
Citrate inhibits phosphofructokinase when tested in vitro past enhancing the inhibitory effect of ATP. However, it is doubtful that this is a meaningful issue in vivo, because citrate in the cytosol is utilized mainly for conversion to acetyl-CoA for fatty acid and cholesterol synthesis.
TIGAR, a p53 induced enzyme, is responsible for the regulation of phosphofructokinase and acts to protect against oxidative stress.[37] TIGAR is a single enzyme with dual part that regulates F2,6BP. It can behave as a phosphatase (fructuose-2,six-bisphosphatase) which cleaves the phosphate at carbon-two producing F6P. Information technology can also behave equally a kinase (PFK2) adding a phosphate onto carbon-2 of F6P which produces F2,6BP. In humans, the TIGAR protein is encoded past C12orf5 factor. The TIGAR enzyme will hinder the forward progression of glycolysis, by creating a build up of fructose-6-phosphate (F6P) which is isomerized into glucose-6-phosphate (G6P). The aggregating of G6P volition shunt carbons into the pentose phosphate pathway.[38] [39]
Pyruvate kinase [edit]

The final footstep of glycolysis is catalysed past pyruvate kinase to grade pyruvate and some other ATP. Information technology is regulated past a range of different transcriptional, covalent and non-covalent regulation mechanisms, which tin can vary widely in unlike tissues.[40] [41] [42] For example, in the liver, pyruvate kinase is regulated based on glucose availability. During fasting (no glucose available), glucagon activates protein kinase A which phosphorylates pyruvate kinase to inhibit information technology.[43] An increase in claret sugar leads to secretion of insulin, which activates poly peptide phosphatase 1, leading to dephosphorylation and re-activation of pyruvate kinase.[43] These controls forbid pyruvate kinase from being active at the same fourth dimension every bit the enzymes that catalyze the reverse reaction (pyruvate carboxylase and phosphoenolpyruvate carboxykinase), preventing a futile bicycle.[43] Conversely, the isoform of pyruvate kinasein found in muscle is not affected by protein kinase A (which is activated by adrenaline in that tissue), so that glycolysis remains active in muscles even during fasting.[43]
Post-glycolysis processes [edit]
The overall procedure of glycolysis is:
- Glucose + 2 NAD+ + two ADP + 2 Pi → two pyruvate + 2 NADH + 2 H+ + two ATP
If glycolysis were to continue indefinitely, all of the NAD+ would exist used up, and glycolysis would stop. To allow glycolysis to go along, organisms must exist able to oxidize NADH back to NAD+. How this is performed depends on which external electron acceptor is available.
Anoxic regeneration of NAD+ [edit]
One method of doing this is to simply have the pyruvate practice the oxidation; in this process, pyruvate is converted to lactate (the conjugate base of lactic acid) in a procedure called lactic acid fermentation:
- Pyruvate + NADH + H+ → lactate + NAD+
This process occurs in the leaner involved in making yogurt (the lactic acrid causes the milk to curdle). This procedure also occurs in animals nether hypoxic (or partially anaerobic) conditions, plant, for example, in overworked muscles that are starved of oxygen. In many tissues, this is a cellular last resort for energy; most animal tissue cannot tolerate anaerobic weather condition for an extended period of fourth dimension.
Some organisms, such every bit yeast, convert NADH dorsum to NAD+ in a procedure called ethanol fermentation. In this procedure, the pyruvate is converted first to acetaldehyde and carbon dioxide, and and so to ethanol.
Lactic acrid fermentation and ethanol fermentation tin occur in the absence of oxygen. This anaerobic fermentation allows many single-cell organisms to use glycolysis as their but energy source.
Anoxic regeneration of NAD+ is merely an effective means of energy production during brusque, intense practise in vertebrates, for a menstruation ranging from 10 seconds to ii minutes during a maximal attempt in humans. (At lower do intensities it can sustain muscle activity in diving animals, such as seals, whales and other aquatic vertebrates, for very much longer periods of time.) Under these weather NAD+ is replenished past NADH donating its electrons to pyruvate to form lactate. This produces 2 ATP molecules per glucose molecule, or nigh v% of glucose's energy potential (38 ATP molecules in leaner). Merely the speed at which ATP is produced in this manner is near 100 times that of oxidative phosphorylation. The pH in the cytoplasm quickly drops when hydrogen ions accrue in the muscle, eventually inhibiting the enzymes involved in glycolysis.
The burning awareness in muscles during difficult exercise tin exist attributed to the release of hydrogen ions during the shift to glucose fermentation from glucose oxidation to carbon dioxide and water, when aerobic metabolism can no longer keep footstep with the free energy demands of the muscles. These hydrogen ions form a office of lactic acid. The trunk falls back on this less efficient but faster method of producing ATP under low oxygen weather condition. This is thought to take been the primary means of energy production in before organisms before oxygen reached high concentrations in the temper between 2000 and 2500 million years ago, and thus would represent a more ancient class of energy production than the aerobic replenishment of NAD+ in cells.
The liver in mammals gets rid of this excess lactate by transforming it back into pyruvate under aerobic weather condition; see Cori cycle.
Fermentation of pyruvate to lactate is sometimes besides called "anaerobic glycolysis", nonetheless, glycolysis ends with the production of pyruvate regardless of the presence or absenteeism of oxygen.
In the to a higher place two examples of fermentation, NADH is oxidized by transferring two electrons to pyruvate. However, anaerobic bacteria apply a wide variety of compounds equally the terminal electron acceptors in cellular respiration: nitrogenous compounds, such every bit nitrates and nitrites; sulfur compounds, such as sulfates, sulfites, sulfur dioxide, and elemental sulfur; carbon dioxide; iron compounds; manganese compounds; cobalt compounds; and uranium compounds.
Aerobic regeneration of NAD+, and disposal of pyruvate [edit]
In aerobic eukaryotes, a circuitous machinery has developed to employ the oxygen in air as the final electron acceptor. Aerobic prokaryotes, which lack mitochondria, use a variety of simpler mechanisms.
- Firstly, the NADH + H+ generated past glycolysis has to be transferred to the mitochondrion to be oxidized, and thus to regenerate the NAD+ necessary for glycolysis to keep. However the inner mitochondrial membrane is impermeable to NADH and NAD+.[44] Apply is therefore made of two "shuttles" to transport the electrons from NADH beyond the mitochondrial membrane. They are the malate-aspartate shuttle and the glycerol phosphate shuttle. In the former the electrons from NADH are transferred to cytosolic oxaloacetate to class malate. The malate and so traverses the inner mitochondrial membrane into the mitochondrial matrix, where it is reoxidized by NAD+ forming intra-mitochondrial oxaloacetate and NADH. The oxaloacetate is so re-cycled to the cytosol via its conversion to aspartate which is readily transported out of the mitochondrion. In the glycerol phosphate shuttle electrons from cytosolic NADH are transferred to dihydroxyacetone to form glycerol-3-phosphate which readily traverses the outer mitochondrial membrane. Glycerol-3-phosphate is then reoxidized to dihydroxyacetone, altruistic its electrons to FAD instead of NAD+.[44] This reaction takes identify on the inner mitochondrial membrane, allowing FADHtwo to donate its electrons directly to coenzyme Q (ubiquinone) which is role of the electron send concatenation which ultimately transfers electrons to molecular oxygen O2 , with the germination of water, and the release of energy somewhen captured in the grade of ATP.
- The glycolytic end-product, pyruvate (plus NAD+) is converted to acetyl-CoA, COii and NADH + H+ inside the mitochondria in a process chosen pyruvate decarboxylation.
- The resulting acetyl-CoA enters the citric acid cycle (or Krebs Cycle), where the acetyl grouping of the acetyl-CoA is converted into carbon dioxide past two decarboxylation reactions with the formation of yet more intra-mitochondrial NADH + H+.
- The intra-mitochondrial NADH + H+ is oxidized to NAD+ by the electron send concatenation, using oxygen as the final electron acceptor to grade water. The free energy released during this procedure is used to create a hydrogen ion (or proton) gradient across the inner membrane of the mitochondrion.
- Finally, the proton slope is used to produce about two.5 ATP for every NADH + H+ oxidized in a process chosen oxidative phosphorylation.[44]
Conversion of carbohydrates into fatty acids and cholesterol [edit]
The pyruvate produced past glycolysis is an of import intermediary in the conversion of carbohydrates into fatty acids and cholesterol.[45] This occurs via the conversion of pyruvate into acetyl-CoA in the mitochondrion. Nevertheless, this acetyl CoA needs to be transported into cytosol where the synthesis of fatty acids and cholesterol occurs. This cannot occur straight. To obtain cytosolic acetyl-CoA, citrate (produced by the condensation of acetyl CoA with oxaloacetate) is removed from the citric acrid cycle and carried across the inner mitochondrial membrane into the cytosol.[45] There it is broken by ATP citrate lyase into acetyl-CoA and oxaloacetate. The oxaloacetate is returned to mitochondrion as malate (then back into oxaloacetate to transfer more acetyl-CoA out of the mitochondrion). The cytosolic acetyl-CoA tin can be carboxylated by acetyl-CoA carboxylase into malonyl CoA, the commencement committed pace in the synthesis of fatty acids, or it can be combined with acetoacetyl-CoA to course 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) which is the rate limiting step controlling the synthesis of cholesterol.[46] Cholesterol tin be used as is, as a structural component of cellular membranes, or information technology can be used to synthesize the steroid hormones, bile salts, and vitamin D.[34] [45] [46]
Conversion of pyruvate into oxaloacetate for the citric acid bicycle [edit]
Pyruvate molecules produced by glycolysis are actively transported beyond the inner mitochondrial membrane, and into the matrix where they can either be oxidized and combined with coenzyme A to form CO2 , acetyl-CoA, and NADH,[34] or they can be carboxylated (by pyruvate carboxylase) to form oxaloacetate. This latter reaction "fills up" the amount of oxaloacetate in the citric acid cycle, and is therefore an anaplerotic reaction (from the Greek significant to "fill"), increasing the cycle's capacity to metabolize acetyl-CoA when the tissue'due south energy needs (due east.g. in heart and skeletal muscle) are suddenly increased by activity.[47] In the citric acid cycle all the intermediates (e.yard. citrate, iso-citrate, blastoff-ketoglutarate, succinate, fumarate, malate and oxaloacetate) are regenerated during each turn of the cycle. Adding more of any of these intermediates to the mitochondrion therefore means that that additional amount is retained within the cycle, increasing all the other intermediates as i is converted into the other. Hence the addition of oxaloacetate profoundly increases the amounts of all the citric acid intermediates, thereby increasing the bicycle's capacity to metabolize acetyl CoA, converting its acetate component into COtwo and water, with the release of enough energy to form 11 ATP and 1 GTP molecule for each additional molecule of acetyl CoA that combines with oxaloacetate in the cycle.[47]
To cataplerotically remove oxaloacetate from the citric cycle, malate can be transported from the mitochondrion into the cytoplasm, decreasing the amount of oxaloacetate that can be regenerated.[47] Furthermore, citric acrid intermediates are constantly used to form a variety of substances such as the purines, pyrimidines and porphyrins.[47]
Intermediates for other pathways [edit]
This article concentrates on the catabolic role of glycolysis with regard to converting potential chemical energy to usable chemic energy during the oxidation of glucose to pyruvate. Many of the metabolites in the glycolytic pathway are also used by anabolic pathways, and, every bit a issue, flux through the pathway is critical to maintain a supply of carbon skeletons for biosynthesis.
The following metabolic pathways are all strongly reliant on glycolysis as a source of metabolites: and many more than.
- Pentose phosphate pathway, which begins with the dehydrogenation of glucose-half dozen-phosphate, the offset intermediate to be produced by glycolysis, produces various pentose sugars, and NADPH for the synthesis of fatty acids and cholesterol.
- Glycogen synthesis also starts with glucose-6-phosphate at the beginning of the glycolytic pathway.
- Glycerol, for the formation of triglycerides and phospholipids, is produced from the glycolytic intermediate glyceraldehyde-3-phosphate.
- Various post-glycolytic pathways:
-
- Fatty acid synthesis
- Cholesterol synthesis
- The citric acrid bicycle which in plough leads to:
-
- Amino acid synthesis
- Nucleotide synthesis
- Tetrapyrrole synthesis
Although gluconeogenesis and glycolysis share many intermediates the one is non functionally a branch or tributary of the other. There are two regulatory steps in both pathways which, when agile in the i pathway, are automatically inactive in the other. The two processes tin can therefore not be simultaneously active.[48] Indeed, if both sets of reactions were highly active at the same time the internet result would be the hydrolysis of four loftier energy phosphate bonds (ii ATP and two GTP) per reaction cycle.[48]
NAD+ is the oxidizing agent in glycolysis, as it is in almost other free energy yielding metabolic reactions (due east.g. beta-oxidation of fatty acids, and during the citric acrid cycle). The NADH thus produced is primarily used to ultimately transfer electrons to O2 to produce h2o, or, when O2 is not available, to produced compounds such as lactate or ethanol (see Anoxic regeneration of NAD+ higher up). NADH is rarely used for synthetic processes, the notable exception being gluconeogenesis. During fatty acrid and cholesterol synthesis the reducing amanuensis is NADPH. This difference exemplifies a general principle that NADPH is consumed during biosynthetic reactions, whereas NADH is generated in free energy-yielding reactions.[48] The source of the NADPH is ii-fold. When malate is oxidatively decarboxylated by "NADP+-linked malic enzyme" pyruvate, COtwo and NADPH are formed. NADPH is also formed by the pentose phosphate pathway which converts glucose into ribose, which can be used in synthesis of nucleotides and nucleic acids, or it can be catabolized to pyruvate.[48]
Glycolysis in disease [edit]
Diabetes [edit]
Cellular uptake of glucose occurs in response to insulin signals, and glucose is subsequently broken downwardly through glycolysis, lowering blood sugar levels. However, the depression insulin levels seen in diabetes consequence in hyperglycemia, where glucose levels in the blood ascent and glucose is not properly taken up by cells. Hepatocytes further contribute to this hyperglycemia through gluconeogenesis. Glycolysis in hepatocytes controls hepatic glucose production, and when glucose is overproduced by the liver without having a ways of existence cleaved down past the torso, hyperglycemia results.[49]
Genetic diseases [edit]
Glycolytic mutations are mostly rare due to importance of the metabolic pathway, this means that the majority of occurring mutations issue in an inability for the cell to respire, and therefore crusade the death of the cell at an early stage. However, some mutations are seen with one notable example being Pyruvate kinase deficiency, leading to chronic hemolytic anemia.
Cancer [edit]
Cancerous tumor cells perform glycolysis at a rate that is x times faster than their noncancerous tissue counterparts.[50] During their genesis, express capillary support often results in hypoxia (decreased O2 supply) within the tumor cells. Thus, these cells rely on anaerobic metabolic processes such as glycolysis for ATP (adenosine triphosphate). Some tumor cells overexpress specific glycolytic enzymes which outcome in college rates of glycolysis.[51] Often these enzymes are Isoenzymes, of traditional glycolysis enzymes, that vary in their susceptibility to traditional feedback inhibition. The increase in glycolytic activeness ultimately counteracts the effects of hypoxia by generating sufficient ATP from this anaerobic pathway.[52] This phenomenon was showtime described in 1930 by Otto Warburg and is referred to equally the Warburg outcome. The Warburg hypothesis claims that cancer is primarily acquired by dysfunctionality in mitochondrial metabolism, rather than because of the uncontrolled growth of cells. A number of theories have been avant-garde to explain the Warburg effect. One such theory suggests that the increased glycolysis is a normal protective process of the body and that cancerous modify could be primarily acquired by energy metabolism.[53]
This high glycolysis rate has important medical applications, every bit high aerobic glycolysis past malignant tumors is utilized clinically to diagnose and monitor handling responses of cancers by imaging uptake of 2-18F-2-deoxyglucose (FDG) (a radioactive modified hexokinase substrate) with positron emission tomography (PET).[54] [55]
There is ongoing research to touch on mitochondrial metabolism and treat cancer by reducing glycolysis and thus starving cancerous cells in various new ways, including a ketogenic nutrition.[56] [57] [58]
Interactive pathway map [edit]
The diagram below shows human protein names. Names in other organisms may exist dissimilar and the number of isozymes (such as HK1, HK2, ...) is probable to be different as well.
Click on genes, proteins and metabolites below to link to corresponding articles. [§ 1]
[[File:

[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]
[[]]

|alt=Glycolysis and Gluconeogenesis edit]]
Glycolysis and Gluconeogenesis edit
- ^ The interactive pathway map tin exist edited at WikiPathways: "GlycolysisGluconeogenesis_WP534".
Alternative nomenclature [edit]
Some of the metabolites in glycolysis take alternative names and nomenclature. In part, this is because some of them are common to other pathways, such as the Calvin cycle.
| This article | Culling | |||
|---|---|---|---|---|
| i | Glucose | Glc | Dextrose | |
| 2 | Glucose-6-phosphate | G6P | ||
| 3 | Fructose-6-phosphate | F6P | ||
| 4 | Fructose-1,6-bisphosphate | F1,6BP | Fructose i,half-dozen-diphosphate | FBP; FDP; F1,6DP |
| v | Dihydroxyacetone phosphate | DHAP | Glycerone phosphate | |
| six | Glyceraldehyde-iii-phosphate | GADP | 3-Phosphoglyceraldehyde | PGAL; G3P; GALP; GAP; TP |
| 7 | 1,three-Bisphosphoglycerate | one,3BPG | Glycerate-1,3-bisphosphate, glycerate-one,3-diphosphate, 1,3-diphosphoglycerate | PGAP; BPG; DPG |
| 8 | iii-Phosphoglycerate | 3PG | Glycerate-3-phosphate | PGA; GP |
| 9 | 2-Phosphoglycerate | 2PG | Glycerate-two-phosphate | |
| x | Phosphoenolpyruvate | PEP | ||
| xi | Pyruvate | Pyr | Pyruvic acid | |
Structure of glycolysis components in Fischer projections and polygonal model [edit]
The intermediates of glycolysis depicted in Fischer projections prove the chemical irresolute stride past step. Such paradigm tin be compared to polygonal model representation.[59] Another comparation of Fischer projections and Poligonal Model in glycolysis is shown in a video.[60] Video animations in the same channel in YouTube can be seen for another metabolic pathway (Krebs Bike) and the representation and applying of Polygonal Model in Organic Chemistry [61]

Glycolysis - Construction of anaerobic glycolysis components showed using Fischer projections, left, and polygonal model, right. The compounds stand for to glucose (GLU), glucose 6-phosphate (G6P), fructose 6-phosphate (F6P), fructose i,half-dozen-bisphosphate ( F16BP), dihydroxyacetone phosphate (DHAP), glyceraldehyde 3-phosphate(GA3P), i,3-bisphosphoglycerate (13BPG), 3-phosphoglycerate (3PG), 2-phosphoglycerate (2PG), phosphoenolpyruvate (PEP), pyruvate (PIR), and lactate (LAC). The enzymes which participate of this pathway are indicated past underlined numbers, and correspond to hexokinase (
ane), glucose-6-phosphate isomerase (
2), phosphofructokinase-1 (
3), fructose-bisphosphate aldolase (
4), triosephosphate isomerase (
5), glyceraldehyde-3-phosphate dehydrogenase (
5), phosphoglycerate kinase (
vii), phosphoglycerate mutase (
8), phosphopyruvate hydratase (enolase) (
9), pyruvate kinase (
10), and lactate dehydrogenase (
11). The participant coenzymes (NAD+, NADH + H+, ATP and ADP), inorganic phosphate,
H2O and
CO2 were omitted in these representations. The phosphorylation reactions from ATP, besides the ADP phosphorylation reactions in later steps of glycolysis are shown every bit ~P respectively entering or going out the pathway. The oxireduction reactions using NAD+ or NADH are observed as hydrogens "2H" going out or inbound the pathway.
See as well [edit]
![]()
Wikimedia Commons has media related to Glycolysis.
- Carbohydrate catabolism
- Citric acid cycle
- Cori cycle
- Fermentation (biochemistry)
- Gluconeogenesis
- Glycolytic oscillation
- Pentose phosphate pathway
- Pyruvate decarboxylation
- Triose kinase
References [edit]
- ^ Alfarouk KO, Verduzco D, Rauch C, Muddathir AK, Adil HH, Elhassan GO, et al. (18 Dec 2014). "Glycolysis, tumor metabolism, cancer growth and broadcasting. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question". Oncoscience. 1 (12): 777–802. doi:10.18632/oncoscience.109. PMC4303887. PMID 25621294.
- ^ Romano AH, Conway T (1996). "Evolution of carbohydrate metabolic pathways". Research in Microbiology. 147 (6–7): 448–455. doi:x.1016/0923-2508(96)83998-2. PMID 9084754.
- ^ Keller MA, Turchyn AV, Ralser M (April 2014). "Non-enzymatic glycolysis and pentose phosphate pathway-like reactions in a plausible Archean sea". Molecular Systems Biology. 10 (4): 725. doi:10.1002/msb.20145228. PMC4023395. PMID 24771084.
- ^ Kim BH, Gadd GM. (2011) Bacterial Physiology and Metabolism, third edition.
- ^ a b c Mehta Due south (20 September 2011). "Glycolysis – Animation and Notes". PharmaXchange.
- ^ a b Lane AN, Fan TW, Higashi RM (2009). "Metabolic acidosis and the importance of balanced equations". Metabolomics. 5 (2): 163–165. doi:10.1007/s11306-008-0142-two. S2CID 35500999.
- ^ Barnett JA (April 2003). "A history of research on yeasts v: the fermentation pathway". Yeast. 20 (6): 509–543. doi:ten.1002/yea.986. PMID 12722184. S2CID 26805351.
- ^ "Louis Pasteur and Alcoholic Fermentation". www.pasteurbrewing.com. Archived from the original on 2011-01-13. Retrieved 2016-02-23 .
- ^ Alba-Lois 50, Segal-Kischinevzky C (January 2010). "Yeast fermentation and the making of beer and wine". Nature Education. three (ix): 17.
- ^ Kohler R (1971-03-01). "The background to Eduard Buchner'south discovery of prison cell-costless fermentation". Journal of the History of Biology. 4 (1): 35–61. doi:x.1007/BF00356976. PMID 11609437. S2CID 46573308.
- ^ "Eduard Buchner - Biographical". www.nobelprize.org . Retrieved 2016-02-23 .
- ^ a b Cornish-Bowden A (1997). "Harden and Immature'south Discovery of Fructose 1,half dozen-Bisphosphate". New Beer in an One-time Canteen: Eduard Buchner and the Growth of Biochemical Knowledge. Valencia, Espana.
- ^ a b Palmer Grand. "Chapter 3: The History of Glycolysis: An Example of a Linear Metabolic Pathway.". Bios 302 (PDF). Archived from the original (PDF) on 18 Nov 2017.
- ^ "Otto Meyerhof - Biographical". world wide web.nobelprize.org . Retrieved 2016-02-23 .
- ^ a b c Kresge Northward, Simoni RD, Hill RL (January 2005). "Otto Fritz Meyerhof and the elucidation of the glycolytic pathway". The Periodical of Biological Chemistry. 280 (4): e3. doi:10.1016/S0021-9258(20)76366-0. PMID 15665335.
- ^ "Embden, Gustav – Dictionary definition of Embden, Gustav | Encyclopedia.com: Complimentary online dictionary". www.encyclopedia.com . Retrieved 2016-02-23 .
- ^ Reeves RE, South DJ, Blytt HJ, Warren LG (December 1974). "Pyrophosphate:D-fructose six-phosphate i-phosphotransferase. A new enzyme with the glycolytic function of half-dozen-phosphofructokinase". The Journal of Biological Chemistry. 249 (24): 7737–7741. doi:10.1016/S0021-9258(19)42029-2. PMID 4372217.
- ^ Selig One thousand, Xavier KB, Santos H, Schönheit P (April 1997). "Comparative analysis of Embden-Meyerhof and Entner-Doudoroff glycolytic pathways in hyperthermophilic archaea and the bacterium Thermotoga". Athenaeum of Microbiology. 167 (four): 217–232. doi:10.1007/BF03356097. PMID 9075622. S2CID 19489719.
- ^ Garrett RH, Grisham CM (2012). Biochemistry (5th ed.). Cengage Learning. ISBN978-i-133-10629-6.
- ^ Berg JM, Tymoczko JL, Stryer Fifty (2007). Biochemistry (sixth ed.). New York: Freeman. p. 622. ISBN978-0716787242.
- ^ a b Garrett R, Grisham CM (2005). Biochemistry (3rd ed.). Belmont, CA: Thomson Brooks/Cole. p. 584. ISBN978-0-534-49033-one.
- ^ Shimizu Grand, Matsuoka Y (March 2019). "Regulation of glycolytic flux and overflow metabolism depending on the source of energy generation for energy need". Biotechnology Advances. 37 (ii): 284–305. doi:x.1016/j.biotechadv.2018.12.007. PMID 30576718. S2CID 58591361.
- ^ a b Chubukov V, Gerosa Fifty, Kochanowski K, Sauer U (May 2014). "Coordination of microbial metabolism". Nature Reviews. Microbiology. 12 (5): 327–340. doi:10.1038/nrmicro3238. PMID 24658329. S2CID 28413431.
- ^ Hochachka PW (1999). Roach RC, Wagner PD, Hackett PH (eds.). "Cross-species studies of glycolytic part". Advances in Experimental Medicine and Biology. Boston, MA: Springer Us. 474: 219–229. doi:ten.1007/978-one-4615-4711-2_18. ISBN978-1-4613-7134-2. PMID 10635004.
- ^ Lemaigre FP, Rousseau GG (October 1994). "Transcriptional command of genes that regulate glycolysis and gluconeogenesis in developed liver". The Biochemical Journal. 303 (1): 1–fourteen. doi:x.1042/bj3030001. PMC1137548. PMID 7945228.
- ^ Bian X, Jiang H, Meng Y, Li YP, Fang J, Lu Z (March 2022). "Regulation of cistron expression past glycolytic and gluconeogenic enzymes". Trends in Cell Biology. 32 (9): 786–799. doi:x.1016/j.tcb.2022.02.003. PMID 35300892. S2CID 247459973.
- ^ a b Gerosa L, Sauer U (Baronial 2011). "Regulation and control of metabolic fluxes in microbes". Current Stance in Biotechnology. 22 (4): 566–575. doi:ten.1016/j.copbio.2011.04.016. PMID 21600757.
- ^ Chowdhury S, Hepper S, Lodi MK, Saier MH, Uetz P (Apr 2021). "The Protein Interactome of Glycolysis in Escherichia coli". Proteomes. ix (2): 16. doi:x.3390/proteomes9020016. PMC8167557. PMID 33917325.
- ^ Rodionova IA, Zhang Z, Mehla J, Goodacre North, Babu M, Emili A, et al. (August 2017). "The phosphocarrier protein HPr of the bacterial phosphotransferase organization globally regulates free energy metabolism by directly interacting with multiple enzymes in Escherichia coli". The Journal of Biological Chemistry. 292 (34): 14250–14257. doi:10.1074/jbc.M117.795294. PMC5572926. PMID 28634232.
- ^ Pisithkul T, Patel NM, Amador-Noguez D (April 2015). "Post-translational modifications as key regulators of bacterial metabolic fluxes". Electric current Opinion in Microbiology. 24: 29–37. doi:x.1016/j.mib.2014.12.006. PMID 25597444.
- ^ a b c Koeslag JH, Saunders PT, Terblanche Eastward (June 2003). "A reappraisal of the claret glucose homeostat which comprehensively explains the type 2 diabetes mellitus-syndrome X circuitous". The Journal of Physiology (published 2003). 549 (Pt 2): 333–346. doi:10.1113/jphysiol.2002.037895. PMC2342944. PMID 12717005.
- ^ a b c d eastward Stryer L (1995). "Glycolysis.". Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. pp. 483–508. ISBN0-7167-2009-four.
- ^ Stryer L (1995). Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. p. 773. ISBN0-7167-2009-four.
- ^ a b c Voet D, Voet JG, Pratt CW (2006). Fundamentals of Biochemistry (2nd ed.). John Wiley and Sons, Inc. pp. 547, 556. ISBN978-0-471-21495-iii.
- ^ Beis I, Newsholme EA (October 1975). "The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates". The Biochemical Journal. 152 (1): 23–32. doi:x.1042/bj1520023. PMC1172435. PMID 1212224.
- ^ Voet D, Voet JG (2004). Biochemistry (3rd ed.). New York: John Wiley & Sons, Inc.
- ^ Lackie J (2010). TIGAR. Oxford Reference Online: Oxford University Press. ISBN9780199549351.
- ^ Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, et al. (July 2006). "TIGAR, a p53-inducible regulator of glycolysis and apoptosis". Cell. 126 (1): 107–120. doi:10.1016/j.prison cell.2006.05.036. PMID 16839880. S2CID 15006256.
- ^ "TIGAR TP53 induced glycolysis regulatory phosphatase [Human being sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov . Retrieved 2018-05-17 .
- ^ Carbonell J, Felíu JE, Marco R, Sols A (Baronial 1973). "Pyruvate kinase. Classes of regulatory isoenzymes in mammalian tissues". European Journal of Biochemistry. 37 (1): 148–156. doi:10.1111/j.1432-1033.1973.tb02969.10. hdl:10261/78345. PMID 4729424.
- ^ Valentini G, Chiarelli L, Fortin R, Speranza ML, Galizzi A, Mattevi A (June 2000). "The allosteric regulation of pyruvate kinase". The Journal of Biological Chemistry. 275 (24): 18145–18152. doi:10.1074/jbc.m001870200. PMID 10751408.
- ^ Israelsen WJ, Vander Heiden MG (July 2015). "Pyruvate kinase: Function, regulation and role in cancer". Seminars in Cell & Developmental Biology. 43: 43–51. doi:10.1016/j.semcdb.2015.08.004. PMC4662905. PMID 26277545.
- ^ a b c d Engström L (1978). "The regulation of liver pyruvate kinase by phosphorylation--dephosphorylation". Current Topics in Cellular Regulation. Elsevier. 13: 28–51. doi:10.1016/b978-0-12-152813-3.50006-nine. ISBN978-0-12-152813-3. PMID 208818.
- ^ a b c Stryer L (1995). "Oxidative phosphorylation.". Biochemistry (Fourth ed.). New York: West.H. Freeman and Visitor. pp. 537–549. ISBN0-7167-2009-4.
- ^ a b c Stryer L (1995). "Fatty acrid metabolism.". Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. pp. 603–628. ISBN0-7167-2009-four.
- ^ a b Stryer L (1995). "Biosynthesis of membrane lipids and steroids.". Biochemistry (Fourth ed.). New York: W.H. Freeman and Visitor. pp. 691–707. ISBN0-7167-2009-4.
- ^ a b c d Stryer L (1995). "Citric acid bike.". Biochemistry (Fourth ed.). New York: W.H. Freeman and Visitor. pp. 509–527, 569–579, 614–616, 638–641, 732–735, 739–748, 770–773. ISBN0-7167-2009-4.
- ^ a b c d Stryer 50 (1995). Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. pp. 559–565, 574–576, 614–623. ISBN0-7167-2009-4.
- ^ Guo X, Li H, Xu H, Woo S, Dong H, Lu F, et al. (2012-08-01). "Glycolysis in the command of claret glucose homeostasis". Acta Pharmaceutica Sinica B. two (four): 358–367. doi:10.1016/j.apsb.2012.06.002. ISSN 2211-3835.
- ^ Alfarouk KO, Verduzco D, Rauch C, Muddathir AK, Adil HH, Elhassan GO, et al. (2014). "Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic arroyo to an old cancer question". Oncoscience. 1 (12): 777–802. doi:10.18632/oncoscience.109. PMC4303887. PMID 25621294.
- ^ Alfarouk KO, Shayoub ME, Muddathir AK, Elhassan Go, Bashir AH (July 2011). "Evolution of Tumor Metabolism might Reverberate Carcinogenesis as a Contrary Development procedure (Dismantling of Multicellularity)". Cancers. iii (3): 3002–3017. doi:10.3390/cancers3033002. PMC3759183. PMID 24310356.
- ^ Nelson DL, Cox MM (2005). Lehninger principles of biochemistry (4th ed.). New York: W.H. Freeman. ISBN978-0-7167-4339-two.
- ^ Gold J (Oct 2011). "What is Cancer?". Archived from the original on May 19, 2018. Retrieved September 8, 2012.
- ^ Pauwels EK, Sturm EJ, Bombardieri E, Cleton FJ, Stokkel MP (Oct 2000). "Positron-emission tomography with [18F]fluorodeoxyglucose. Function I. Biochemical uptake mechanism and its implication for clinical studies". Periodical of Cancer Research and Clinical Oncology. 126 (x): 549–59. doi:10.1007/pl00008465. PMID 11043392. S2CID 2725555.
- ^ "PET Scan: PET Scan Info Reveals ..." Retrieved December 5, 2005.
- ^ Schwartz 50, Seyfried T, Alfarouk KO, Da Veiga Moreira J, Fais S (Apr 2017). "Out of Warburg issue: An effective cancer treatment targeting the tumor specific metabolism and dysregulated pH". Seminars in Cancer Biology. 43: 134–138. doi:ten.1016/j.semcancer.2017.01.005. PMID 28122260.
- ^ Schwartz L, Supuran CT, Alfarouk KO (2017). "The Warburg Consequence and the Hallmarks of Cancer". Anti-Cancer Agents in Medicinal Chemistry. 17 (2): 164–170. doi:10.2174/1871520616666161031143301. PMID 27804847.
- ^ Maroon J, Bost J, Amos A, Zuccoli G (Baronial 2013). "Restricted calorie ketogenic diet for the handling of glioblastoma multiforme". Journal of Child Neurology. 28 (8): 1002–1008. doi:10.1177/0883073813488670. PMID 23670248. S2CID 1994087.
- ^ Bonafe CF, Bispo JA, de Jesus MB (Jan 2018). "The polygonal model: A uncomplicated representation of biomolecules as a tool for teaching metabolism". Biochemistry and Molecular Biology Education. 46 (1): 66–75. doi:10.1002/bmb.21093. PMID 29131491. S2CID 31317102.
- ^ Bonafe C (23 September 2019). "Introduction to Polygonal Model - PART 1. Glycolysis and Structure of the Participant Molecules". YouTube. Archived from the original on 2021-11-04.
- ^ "Metabolism Blitheness and Polygonal Model". YouTube . Retrieved 2019-12-11 .
External links [edit]
- A Detailed Glycolysis Blitheness provided by IUBMB (Adobe Flash Required)
- The Glycolytic enzymes in Glycolysis at RCSB PDB
- Glycolytic cycle with animations at wdv.com
- Metabolism, Cellular Respiration and Photosynthesis - The Virtual Library of Biochemistry, Molecular Biology and Prison cell Biology
- The chemic logic behind glycolysis at ufp.pt
- Expasy biochemical pathways poster at ExPASy
- MedicalMnemonics.com: 317 5468
- metpath: Interactive representation of glycolysis
Substrate Level Phosphorylation Occurs In,
Source: https://en.wikipedia.org/wiki/Glycolysis
Posted by: kelleywoming.blogspot.com



0 Response to "Substrate Level Phosphorylation Occurs In"
Post a Comment